The complete guide to cleanroom solutions for pharmaceutical manufacturing
Cleanrooms. From stick-built cleanroom systems to modular cleanroom solutions. From hard wall to soft wall cleanroom variants. From dry rooms to clean zones. From ISO standards to Federal Standards in the United States and EU-specific GMP guidelines. There’s a world of concepts, processes, techniques, and standards you must be familiar with before you get yourself a cleanroom solution
And when it comes to cleanroom solutions for pharmaceutical manufacturing? Standard practices and cleanliness requirements are that much higher, given the high stakes involved. In other words, when people’s health is at stake, there is no room for error; there is no tolerance for shortcuts or compromises
This blog series is your ultimate guide to cleanroom solutions for pharmaceutical manufacturing. It covers all you need to know before you partner up with a cleanroom manufacturer and invest in a solution for your pharma production needs.
Considering the fact that the design, construction, and operation of cleanroom systems are governed in large part by relevant standards and regulations in different parts of the world, in this blog series, we’ll spend time understanding what these standards, grades, and classifications are. At the end of the day, compliance with these standards is what will form the foundation of a safe and effective cleanroom solution for your pharmaceutical manufacturing needs.
Ensuring the quality of pharmaceutical products through cleanroom control.
The Food and Drug Administration (FDA) in the United States, Health Canada in their northern neighbour, or the relevant authority (often a national FDA) oversee the safe and efficacious production of pharmaceutical products in different countries. They do this by instituting precise and stringent regulations that drugmakers must abide by.
Good Manufacturing Practices (GMP) are a product of this regulatory framework.
Current Good Manufacturing Practices (cGMP) guidelines, which also encompass cleanroom systems, have been designed to ensure product safety, efficacy and purity. They are designed to minimise contamination risks from microorganisms, particulate matter, pyrogens, and other types of contaminants, particularly during sterile manufacturing processes, including pharmaceutical product preparation, filling and packaging.
Cleanroom system GMP are a form of not only maintaining consumer safety but also consumer trust. Beyond product safety, they were instituted to ensure that pharmaceutical products do indeed contain the ingredients and quantities that they claim to be made up of.
On the whole, in the pharmaceutical manufacturing industry, GMP guidelines cover everything from production processes and quality control to personnel, packaging, and facility specifications. When it comes specifically to cleanroom systems, cleanroom GMP requirements cover facility design, environmental controls, personnel training and behaviour, cleaning procedures, waste disposal methods, and more.
In keeping with GMP guidelines for cleanrooms, there are cleanroom classification systems. These systems constitute a structured method for defining cleanliness levels within cleanroom solutions meant for pharma manufacturing. They define acceptable contaminant levels based on microbial control and airborne particulate concentration.
Largely, two major standards are followed widely around the world. For starters, there’s the ISO 14644-1 standard: this guideline defines the specifics of cleanroom classes from ISO Class 5 to ISO Class 9 to maintain predetermined acceptable levels of airborne particulate concentration. Then there’s also the EU GMP Annexe 1, which grades cleanroom systems from Grade A to Grade D. This guideline applies ISO principles to pharmaceutical environments, while also adding gowning expectations, microbial limits, operational state distinctions and more over and above the ISO requirements.
So, let’s dive in. We’re going to explore the features of cleanroom solutions, the different types of cleanrooms, the different classification and grading systems for cleanroom solutions, and more.
What is a cleanroom?
Cleanroom systems are controlled environments specifically designed to minimise the presence of airborne particulate contamination, pollutants, and other undesirable materials. Cleanroom solutions are designed to maintain very, very low levels of airborne particles, and you can understand why – when you’re manufacturing sterile pharmaceutical products (or even, say, semiconductors, for that matter), it’s only natural that the space in which production occurs is also sterile or ultra-clean.
We’re focusing only on the application of cleanroom solutions to pharma manufacturing today, but cleanroom systems are widely used in diverse industries: biotechnology, aerospace engineering, semiconductor manufacturing, scientific research, and beyond.
Regardless of their specific application, cleanroom solutions generally have some shared characteristics. They house advanced air filtration systems like HEPA or ULPA filters that continuously purify the air of airborne contaminants. When it comes to construction, cleanroom manufacturers use specialised construction materials for the flooring, walls, ceilings, doors, and windows: these construction materials must be smooth, easy-to-clean, non-shedding, and non-emitting surfaces to limit the number of contaminants that collect on them or emanate from them.
Finally, personnel: regardless of the industry, cleanroom personnel must be well-trained and knowledgeable. Their behaviour and activity must be governed by strict protocols, including gowning procedures, handwashing procedures, etc. Since human error is the easiest way for contaminants to enter the cleanroom system, this last factor is the most critical to the success of your cleanroom solution.
Okay, so there are some cleanroom classifications. Do they really matter?
How much do cleanroom classifications really matter?
Having the appropriate classification for your cleanroom and adhering to it strictly is of utmost importance. Not only to ensure regulatory compliance, but also because the quality and safety of the products you make are directly dependent on it.
When your cleanroom solution has the correct classification and you strictly meet all the defined requirements, you:
- Avoid penalties or loss of certification that may come with violating mandated guidelines.
- Prevent product defects that can occur in the presence of even one unwanted particle in the cleanroom system.
- Mitigate the risk of expensive production delays, product recalls, and any other hitch that can set you back, in terms of time and money.
As you can see, choosing the wrong cleanroom class or not complying in full with the requirements of your chosen class could lead to contamination, financial losses, compliance violations and ultimately, harm to your business’s products and reputation. Given the risks, understanding these widely accepted standards is non-negotiable.
So that’s exactly what we’re going to do in part two of this blog post: we’re going to sneak a glimpse into what ISO 14644 is, what it means for your cleanroom system to be classified ISO 5 or ISO 9, and what it means for a space in your facility to be graded A, B, C or D.
While all cleanroom systems are held to a certain standard, your specific cleanroom solution will vary from others based on several factors. The industry you’re in, the product that you’re researching or manufacturing, the kinds of agents you work with and the risks they pose – these are a few of the factors that will decide the grade and/or class of your cleanroom solution.
Let’s understand these classification systems and standards so that you can apply them appropriately to your facility.
What is ISO 14644?
Governed by the International Organisation for Standardisation (ISO), ISO 14644 is a family of international standards that governs cleanrooms and related controlled environments. It encompasses the classification, design, testing and operation of such spaces.
It was first published in 1999 as a replacement for the US Federal Standard 209E. This globally recognised system takes a more modern approach to airborne particulate control, using the internationally applicable metric-based system. Over the years, ISO 14644 has evolved to expand into multiple parts, with each part addressing a particulate aspect of cleanroom performance.
For starters, ISO 14644-1 outlines the classification of spaces by airborne particle concentration. It covers the nine ISO classes, from ISO 1, i.e. the cleanest type of cleanroom, to ISO 9, i.e. the least stringently controlled cleanroom system. Each of these classes is defined based on the maximum allowable number of particles per cubic metre for different particle sizes.
ISO 14644-2 defines requirements for the monitoring and maintenance of air cleanliness over time. This includes alarm thresholds, routine testing, requalification frequency based on risk, and more. This part of the standard ensures that cleanliness requirements are met not only on the establishment of the facility but also continuously during operations, throughout the lifecycle of the cleanroom system.
ISO 14644-3 outlines the testing methods for pressure differentials, air velocity, filter integrity, airflow visualisation, and more.
ISO 14644-4? That’s all about the design and construction of your cleanroom solution. Think HVAC systems, surface materials, layout planning, etc.
ISO 14644-5 is about the operational aspects of your cleanroom systems. Cleaning procedures, personnel behaviour, gowning protocols, and contamination control methods during active manufacturing.
ISO 14644-6 covers cleanroom terminology.
ISO 14644-7 covers separative devices like isolators and RABS (Restricted Access Barrier Systems).
ISO 14644-8 is all about airborne molecular contamination.
ISO 14644-9 is about surface cleanliness in terms of particle concentration.
ISO 14644-10 is about surface cleanliness in terms of chemical concentration.
Put together, this standard offers a complete guide, a structured framework on how to build and operate a cleanroom system. And since it’s consistent and globally accepted, it makes regulatory compliance in new geographies, new product categories, and different markets that much easier.
ISO cleanroom classification under ISO 14644-1
Under ISO 14644-1, cleanrooms are classified based on the number and size of particles permissible per cubic metre of air. This table specifies the maximum values for every class:
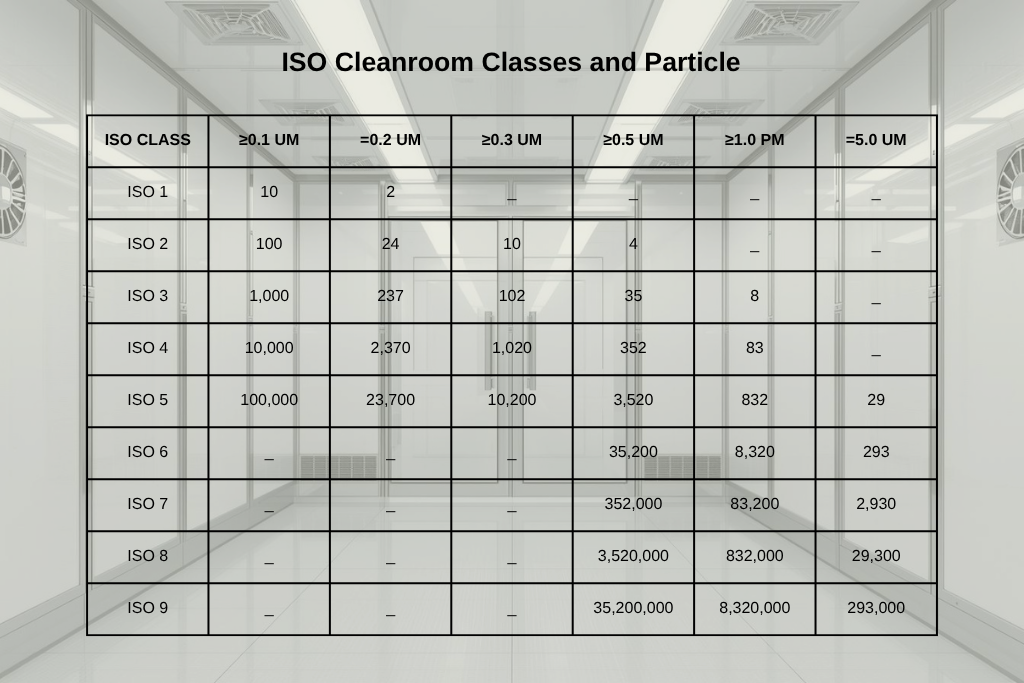
ISO Class 1 is the highest standard of cleanliness among cleanroom classifications; it is ultra-sterile, serving as the pinnacle of controlled environments. ISO 1 cleanroom solutions are used in nanotechnology, semiconductor fabrication, biotech research and other applications which require extremely high levels of purity and precision.
ISO Class 2 cleanroom solutions adhere to standards slightly less stringent than ISO 1. They are used for applications like sophisticated optics and specialised biotech research that involve sensitive processes and call for minimal contamination while maintaining high precision.
Next in terms of required cleanliness levels comes ISO 3, suitable for pharmaceutical manufacturing, aerospace engineering, and some biotech sectors.
ISO 4 is typically used for electronics assembly, optics, and the manufacturing of some pharmaceutical products.
ISO 5 is usually reserved for electronics manufacturing and some medical device production, among other applications. ISO 5 cleanrooms are not as sterile as ISO 1 to 4, but they still offer a very controlled environment that allows manufacturers to maintain product quality and meet industry standards without taking on the financial and operational burden of more strictly controlled spaces.
ISO 6 cleanrooms are used in industries and applications with a slightly higher particle tolerance. They are essential for food processing, certain lab processes, and certain manufacturing processes.
ISO 7, 8 and 9 cleanrooms are increasingly more tolerant of particle presence, but they are still controlled environments. ISO 9 cleanrooms are usually used for general assembly lines, standard manufacturing processes, and the like.
Grades A, B, C and D for cleanroom facilities
The EU GMP Annexe 1 applies the ISO classifications and principles to pharmaceutical settings. It also adds gowning expectations, microbial limits, and distinctions between different operational states, i.e. in operation and at rest.
Let’s look more closely at some of the details of this grading system.
Not all drugs need to be manufactured in a cleanroom. Some drugs, like non-sterile pharmaceuticals, can be produced in environments with much higher particle tolerance. The aforementioned GMP grades define the environment in which sterile drugs and biological products must be manufactured.
GMP cleanrooms and associated spaces are classed into four grades, from A to D. Each grade indicates how critical the operations performed in that area are. It is important to note that each of these grades correlates with an ISO class. Thus, this isn’t an unconnected system; rather, it is a closely related and harmonised system with the ISO classification standard.
Before we get into the specifics, however, you must understand two important terminologies used in this grading system: “at rest” and “in operation”. While “at rest” refers to the state in which the cleanroom system simply is, “in operation” refers to the state of full cleanroom functionality, with HVAC fully engaged, equipment in use, and the maximum number of personnel engaged in their routine work. In other words, this state reflects real-world manufacturing conditions.
Grade A spaces are meant for the most critical operations in pharmaceutical manufacturing. Think aseptic compounding, aseptic filling, aseptic assembly of filling and mixing, open handling of sterile products, loading a lyophiliser, etc. A grade A space is equivalent to an ISO 5 cleanroom, for both at rest and in operation states
Grade B spaces are intended as background environments for Grade A areas. They support aseptic processing. In operation, a grade B cleanroom is equivalent to an ISO 7 cleanroom. At rest, it is equivalent to ISO 5.
Grade C areas are meant for preparing solutions, handling items before sterilisation, etc. – basically, the less critical stages of the pharma manufacturing process. At rest, this is equivalent to ISO 7. In operation, it is ISO 8.
Grade D, finally, is designated to the spaces that house the least critical stages of sterile pharma manufacturing. Think initial material handling, washing components, cleaning equipment, assembling equipment and accessories before sterilisation, etc. At rest, a grade D space is equivalent to an ISO 8 cleanroom. In operation, the classification of this space is not predefined because it will depend on the nature of the processes you carry out in this area.
Modern cleanroom solutions for pharmaceutical manufacturing
The demand for cleanrooms for pharmaceutical manufacturing has been growing in recent years. Almost every facility producing pharmaceuticals or biologics has at least one cleanroom. As demand has evolved, so have the technologies used to design and construct cleanroom systems.
While stick-built cleanroom systems are still the primary type of cleanroom solution, prefabricated cleanrooms and modular cleanroom solutions are gaining popularity, and with good reason. Modular cleanroom solutions offer technological and operational advantages that traditionally constructed cleanrooms just can’t compete with.
At PodTech™, we want to make biopharma capacity building as easy as pie… accessible to everyone who needs it. That’s why we bring modular cleanroom situations to customers around the world. Speed, built-in quality control, integrated HVAC and utility distribution, sustainability, flexibility, scalability… the list of benefits of our modular cleanroom solutions is a long one. Reach out to us today to know how they can fit your needs.
